Technology Development | Subversive Achievements of Professor Hu Xiaojun of Zhejiang University of Technology
Release time:
Apr 27,2022
The team of Professor Hu Xiaojun of Zhejiang University of Technology innovatively "restored" the growth process of chemical vapor deposition diamond, and realized the point "stone" into "drill" under low pressure, which provided a new strategy and theoretical basis for the synthesis of large-area diamond. When it comes to diamonds, many people first think of dazzling diamonds. In fact, the application of synthetic diamond in industrial production is also "dazzling". It has all the excellent properties of natural diamonds and is widely used in precision cutting tools, wear-resistant devices, semiconductors and electronic devices, low magnetic detection, biomedicine and so on. At present, there are two main types of industrial synthesis of synthetic diamond: high pressure and high temperature method and chemical vapor deposition method. However, due to the limitation of high temperature and high pressure equipment, it is still difficult to prepare large-size single crystal diamond; chemical vapor deposition needs to grow single crystal diamond with natural single crystal diamond as the substrate, and natural single crystal diamond is limited by area, still can not prepare large-area diamond, which greatly limits the application of artificial diamond. The team of Professor Hu Xiaojun of Zhejiang University of Technology has long focused on the research work of diamond films and nano-carbon materials, and is committed to exploring the preparation, doping new methods and photoelectric properties of diamond films and other materials. The research team is concerned that compared with graphite, the thermodynamically metastable diamond can be formed under the low pressure of chemical vapor deposition, and its unique formation mechanism may contain a way to synthesize large-area diamond. However, the growth environment of chemical vapor deposition is complex and it is difficult to achieve in-situ characterization, so the formation mechanism of diamond in the deposition process has always been a difficult problem for scientists in the field of materials. Hu Xiaojun's team used the slow growth method to "restore" the growth process of chemical vapor deposition diamond. The team used "cauliflower"-shaped nano-diamond particles as templates and adopted a series of short-term growth strategies to form instantaneous thin layers. Through direct observation of scanning electron microscopy, Raman spectroscopy and high-resolution transmission electron microscopy, the surface morphology and microstructure of a series of thin layers grown on the "cauliflower"-shaped template at short intervals of 30 seconds at a growth power of 1800 watts were obtained, it was found that the nano-diamond matrix-the initial growth of erect graphene-the growth of erect graphene-the bending of erect graphene into needle-like graphite-the disappearance of needle-like graphite-the recovery of the nano-diamond matrix cycle. This is the first time that the cyclic appearance of graphite/diamond has been found in the chemical vapor deposition process. So how does this process occur? One conjecture is that graphite and diamond grow in turn, and the diamond is covered with graphite after it grows; if so, a large amount of graphite should still be observed in the Raman spectrum after diamond formation, but the actual situation is that the Raman characteristics of the sample are typical of nanodiamond films; To further confirm this never-before-reported phenomenon and the bold conjecture that graphite turns into diamond, the team reduced the growth power to 1600 watts and extended the growth time to 12 minutes to slow the growth rate to capture clearer evidence of graphite turning into diamond. In the 4-minute sample, the main component is relatively straight graphene (Fig. 4b), which changes to graphite nanoneedles (Fig. 4d) at 8 minutes. This nanoneedle contains both graphite (002) and diamond (111) facets (Fig. 4d). When the time was extended to 12 minutes, the graphite disappeared completely, and a large number of diamond grains were observed in the sample (Fig. 4f), indicating that the graphite had been completely converted into diamond. This indicates that the 8 min sample with both diamond (111) and graphite (002) crystal planes is an intermediate transition state for the conversion of graphite to diamond. Further analysis of the structural evolution of this transition state (Figure 4g) shows that graphite (002) is found in the head region 1 of the sample in 8 minutes, a new and darker diamond crystal plane (0.21 nm) appears in the middle region 2 covering the graphite (002) crystal lattice, and the diamond crystal plane (0.21 nm) in the middle region 3 enhances the graphite (002) to weaken, in the root region 4, the graphite (002) lattice disappears and the diamond (0.21 nm) lattice becomes the host lattice. This clearly demonstrates the gradual conversion of graphite to diamond, as shown in Schemes 4j and j-1. It can be seen that in the process of chemical vapor deposition, the formation of diamond is from the phase change of graphite, which subverts the traditional concepts of "active carbon atoms piled up into sp3 diamond lattice" and "sp2 graphite carbon phase is the 'carbon rubbish' in the growth process of diamond film, which is removed by hydrogen etching in the atmosphere.
 The team of Professor Hu Xiaojun of Zhejiang University of Technology innovatively "restored" the growth process of chemical vapor deposition diamond, and realized the point "stone" into "drill" under low pressure, which provided a new strategy and theoretical basis for the synthesis of large-area diamond.
The team of Professor Hu Xiaojun of Zhejiang University of Technology innovatively "restored" the growth process of chemical vapor deposition diamond, and realized the point "stone" into "drill" under low pressure, which provided a new strategy and theoretical basis for the synthesis of large-area diamond.The team of Professor Hu Xiaojun of Zhejiang University of Technology has long focused on the research work of diamond films and nano-carbon materials, and is committed to exploring the preparation, doping new methods and photoelectric properties of diamond films and other materials. The research team is concerned that compared with graphite, the thermodynamically metastable diamond can be formed under the low pressure of chemical vapor deposition, and its unique formation mechanism may contain a way to synthesize large-area diamond. However, the growth environment of chemical vapor deposition is complex and it is difficult to achieve in-situ characterization, so the formation mechanism of diamond in the deposition process has always been a difficult problem for scientists in the field of materials..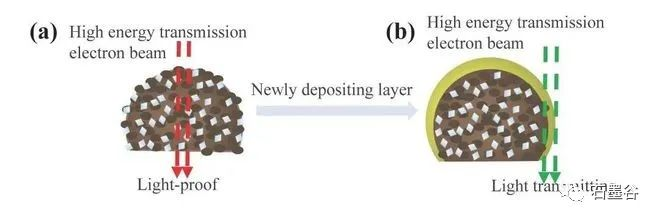
Hu Xiaojun's team used the slow growth method to "restore" the growth process of chemical vapor deposition diamond.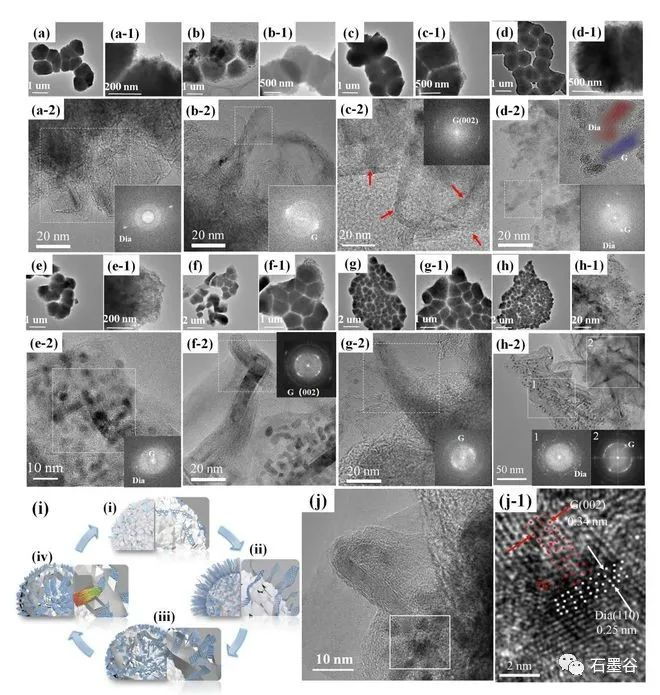 The team used "cauliflower"-shaped nano-diamond particles as templates and adopted a series of short-term growth strategies to form instantaneous thin layers. Through direct observation of scanning electron microscopy, Raman spectroscopy and high-resolution transmission electron microscopy, the surface morphology and microstructure of a series of thin layers grown on the "cauliflower"-shaped template at short intervals of 30 seconds at a growth power of 1800 watts were obtained, it was found that the nano-diamond matrix-the initial growth of erect graphene-the growth of erect graphene-the bending of erect graphene into needle-like graphite-the disappearance of needle-like graphite-the recovery of the nano-diamond matrix cycle.
The team used "cauliflower"-shaped nano-diamond particles as templates and adopted a series of short-term growth strategies to form instantaneous thin layers. Through direct observation of scanning electron microscopy, Raman spectroscopy and high-resolution transmission electron microscopy, the surface morphology and microstructure of a series of thin layers grown on the "cauliflower"-shaped template at short intervals of 30 seconds at a growth power of 1800 watts were obtained, it was found that the nano-diamond matrix-the initial growth of erect graphene-the growth of erect graphene-the bending of erect graphene into needle-like graphite-the disappearance of needle-like graphite-the recovery of the nano-diamond matrix cycle.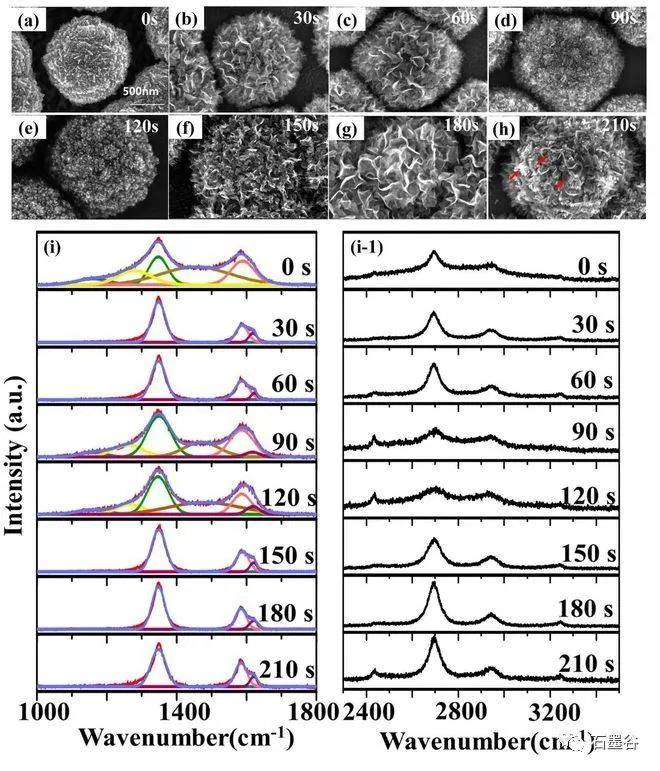
To further confirm this never-before-reported phenomenon and the bold conjecture that graphite turns into diamond, the team reduced the growth power to 1600 watts and extended the growth time to 12 minutes to slow the growth rate to capture clearer evidence of graphite turning into diamond.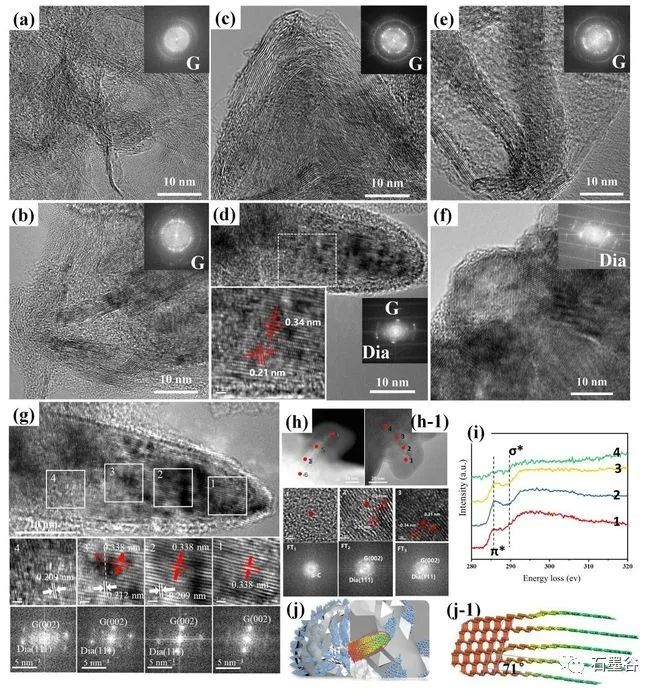
In the 4-minute sample, the main component is relatively straight graphene (Fig. 4b), which changes to graphite nanoneedles (Fig. 4d) at 8 minutes. This nanoneedle contains both graphite (002) and diamond (111) facets (Fig. 4d). When the time was extended to 12 minutes, the graphite disappeared completely, and a large number of diamond grains were observed in the sample (Fig. 4f), indicating that the graphite had been completely converted into diamond. This indicates that the 8 min sample with both diamond (111) and graphite (002) crystal planes is an intermediate transition state for the conversion of graphite to diamond.
Related News






