Graphene modified diaphragm eliminates local temperature hot spots and stabilizes lithium metal anode
Release time:
May 30,2022
Lithium-ion battery is an advanced electrochemical energy storage technology for portable electronic devices and electric vehicles. However, the traditional lithium-ion battery with graphite as the negative electrode has a low specific capacity and energy density is close to the limit, which makes it difficult to meet the demand for high energy density secondary batteries. Lithium metal anode is regarded as a very competitive candidate material for achieving high energy density secondary batteries due to its ultra-high theoretical specific energy and lowest electrochemical potential. However, in the actual situation, lithium metal due to its high electrochemical activity and tend to dendrite morphology of uneven deposition characteristics will greatly shorten the service life of the battery, resulting in thermal runaway and other safety issues. Recently, Professor Tang Wei's team from the School of Chemical Engineering of Xi 'an Jiaotong University, Professor Liu Zhaolin from Singapore's A * STAR Institute of Materials Engineering, and Xie Jingying, chief researcher of Shanghai Space Power Research Institute, established a heat transfer-electrochemical deposition coupling model to investigate the space-time evolution of heating power, temperature and lithium ion distribution of lithium deposition system under different deposition currents and overpotentials. The model results show that there are local temperature hot spots at the tips of lithium dendrites, and the existence of local hot spots aggravates the uneven local lithium deposition, which further promotes the growth of lithium dendrites. By introducing graphene sheet coating diaphragm as an in-situ thermal dispersion medium to eliminate local temperature hot spots, the growth of dendrites can be effectively inhibited, and uniform and dense deposition morphology and efficient and stable cycle can be achieved. The composite diaphragm lithium-copper half-cell achieves a stable cycle of 95% coulombic efficiency and more than 240 cycles at a current density of 1 mA cm-2. The lithium metal electrode cycled under the regular PP diaphragm can be "restored" by the composite diaphragm to a stable and efficient cycle with a coulomb efficiency of more than 95% and a more uniform lithium deposition morphology after the cycle coulomb efficiency is reduced to about 60%. In addition, the composite separator achieves stable cycling, high capacity retention and "recovery" characteristics in a Li | | NCM811 battery with a 30.06 mg cm-2 ultra-high load cathode (3.3 low N/P ratio). The article was published in the top international journal Advanced Energy Materials. 1. Simulation of thermodynamic properties of lithium deposition The coupled heat transfer-electrochemical deposition model reveals the local temperature hot spots of the uneven lithium deposition tip under different deposition overpotentials and current densities. The original defects on the surface of the lithium metal electrode lead to non-uniform distribution of the electric field, causing local concentration of lithium ion flux and large reaction current density, resulting in preferential deposition of lithium ions in the tip region and accompanied by ultra-high heat generation rate. The high rate of heat generation and the low thermal conductivity of conventional liquid electrolytes and polymer separators result in significant localized temperature hotspots at the dendrite tips. 2. High thermal conductivity composite diaphragm Excellent single-layer/few-layer graphene dispersion can be obtained by electrochemical exfoliation method and a composite membrane with layered stacked graphene layers covering commercial membranes can be obtained by simple vacuum filtration method. The ion transport characteristics and mechanical strength of the composite separator remain good compared to the original separator, and the wettability and in-plane thermal conductivity are greatly improved. 3. Lithium deposition characteristics and electrochemical performance The structural characteristics of the commercial PP separator and the original defects on the electrode surface cause the lithium deposition to tend to form dendrites and generate local temperature hot spots, which in turn accelerate the aggregation of lithium ions at the tip of the dendrite lithium deposition to further intensify the growth of lithium dendrites. The high thermal conductivity graphene layer on the surface of the composite diaphragm can spread the accumulated heat in time, effectively avoiding the deterioration of dendrites. A large number of irregular dendritic lithium deposits were observed on the surface of the copper current collector after cycling of the blank separator half-cell. In contrast, the composite separator half-cell achieved a relatively uniform deposition morphology. Therefore, the composite separator battery can achieve long cycle stability of more than 240 weeks at a current density of 1 mA cm-2, with a CE of more than 95%. In addition, the lithium metal electrode with degraded performance after cycling under the blank separator can "recover" to better surface morphology and cycling stability under the composite separator. 4. Full battery performance of lithium metal batteries The effectiveness of the high thermal conductivity diaphragm to eliminate local temperature hot spots to suppress lithium dendrites was further verified by the NCM811 full battery. The composite separator not only achieves more stable cycling and capacity retention in regular surface load positive electrode batteries, but also achieves better capacity retention and "recovery" characteristics when matched with an ultra-high surface capacity positive electrode of 30.06 mg cm-2(3.3 low N/P ratio). The evolution of heat generation rate around lithium dendrites and its relationship with local dendrite growth were investigated based on a coupled electrochemical deposition-heat transfer model. Local rapid electrochemical deposition can easily lead to the accumulation of heat and the generation of temperature hot spots, which leads to the subsequent rapid growth of lithium dendrites, which in turn forms a more serious hot spot problem. The introduction of high thermal conductivity graphene layer on the surface of the diaphragm as an in-situ thermal diffusion medium can effectively eliminate the temperature hot spot, resolve the potential risk of rapid deterioration of dendrites, and obtain a more uniform lithium deposition morphology and stable and efficient electrochemical performance. This study provides a unique thermodynamic perspective for the in-depth understanding of the growth and evolution of lithium dendrites, and paves the way for the effective protection of lithium metal anodes and the practical application of lithium metal secondary batteries.
Lithium-ion battery is an advanced electrochemical energy storage technology for portable electronic devices and electric vehicles. However, the traditional lithium-ion battery with graphite as the negative electrode has a low specific capacity and energy density is close to the limit, which makes it difficult to meet the demand for high energy density secondary batteries. Lithium metal anode is regarded as a very competitive candidate material for achieving high energy density secondary batteries due to its ultra-high theoretical specific energy and lowest electrochemical potential. However, in the actual situation, lithium metal due to its high electrochemical activity and tend to dendrite morphology of uneven deposition characteristics will greatly shorten the service life of the battery, resulting in thermal runaway and other safety issues.
Recently, Professor Tang Wei's team from the School of Chemical Engineering of Xi 'an Jiaotong University, Professor Liu Zhaolin from Singapore's A * STAR Institute of Materials Engineering, and Xie Jingying, chief researcher of Shanghai Space Power Research Institute, established a heat transfer-electrochemical deposition coupling model to investigate the space-time evolution of heating power, temperature and lithium ion distribution of lithium deposition system under different deposition currents and overpotentials. The model results show that there are local temperature hot spots at the tips of lithium dendrites, and the existence of local hot spots aggravates the uneven local lithium deposition, which further promotes the growth of lithium dendrites. By introducing graphene sheet coating diaphragm as an in-situ thermal dispersion medium to eliminate local temperature hot spots, the growth of dendrites can be effectively inhibited, and uniform and dense deposition morphology and efficient and stable cycle can be achieved. The composite diaphragm lithium-copper half-cell achieves a stable cycle of 95% coulombic efficiency and more than 240 cycles at a current density of 1 mA cm-2. The lithium metal electrode cycled under the regular PP diaphragm can be "restored" by the composite diaphragm to a stable and efficient cycle with a coulomb efficiency of more than 95% and a more uniform lithium deposition morphology after the cycle coulomb efficiency is reduced to about 60%. In addition, the composite separator achieves stable cycling, high capacity retention and "recovery" characteristics in a Li | | NCM811 battery with a 30.06 mg cm-2 ultra-high load cathode (3.3 low N/P ratio). The article was published in the top international journal Advanced Energy Materials.

1. Simulation of thermodynamic properties of lithium deposition
The coupled heat transfer-electrochemical deposition model reveals the local temperature hot spots of the uneven lithium deposition tip under different deposition overpotentials and current densities. The original defects on the surface of the lithium metal electrode lead to non-uniform distribution of the electric field, causing local concentration of lithium ion flux and large reaction current density, resulting in preferential deposition of lithium ions in the tip region and accompanied by ultra-high heat generation rate. The high rate of heat generation and the low thermal conductivity of conventional liquid electrolytes and polymer separators result in significant localized temperature hotspots at the dendrite tips.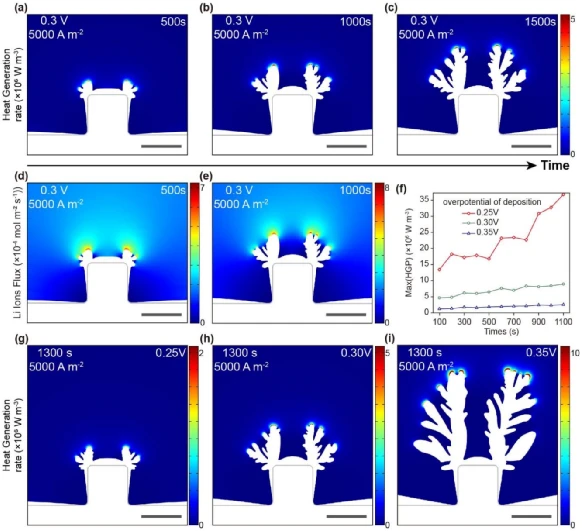
2. High thermal conductivity composite diaphragm
Excellent single-layer/few-layer graphene dispersion can be obtained by electrochemical exfoliation method and a composite membrane with layered stacked graphene layers covering commercial membranes can be obtained by simple vacuum filtration method. The ion transport characteristics and mechanical strength of the composite separator remain good compared to the original separator, and the wettability and in-plane thermal conductivity are greatly improved.

3. Lithium deposition characteristics and electrochemical performance
The structural characteristics of the commercial PP separator and the original defects on the electrode surface cause the lithium deposition to tend to form dendrites and generate local temperature hot spots, which in turn accelerate the aggregation of lithium ions at the tip of the dendrite lithium deposition to further intensify the growth of lithium dendrites. The high thermal conductivity graphene layer on the surface of the composite diaphragm can spread the accumulated heat in time, effectively avoiding the deterioration of dendrites. A large number of irregular dendritic lithium deposits were observed on the surface of the copper current collector after cycling of the blank separator half-cell. In contrast, the composite separator half-cell achieved a relatively uniform deposition morphology. Therefore, the composite separator battery can achieve long cycle stability of more than 240 weeks at a current density of 1 mA cm-2, with a CE of more than 95%. In addition, the lithium metal electrode with degraded performance after cycling under the blank separator can "recover" to better surface morphology and cycling stability under the composite separator.
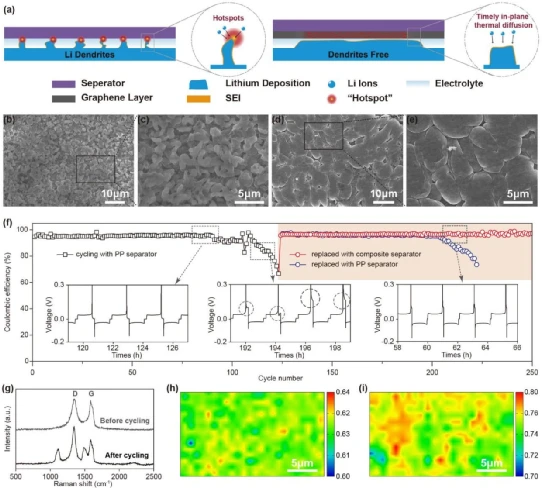
4. Full battery performance of lithium metal batteries
The effectiveness of the high thermal conductivity diaphragm to eliminate local temperature hot spots to suppress lithium dendrites was further verified by the NCM811 full battery. The composite separator not only achieves more stable cycling and capacity retention in regular surface load positive electrode batteries, but also achieves better capacity retention and "recovery" characteristics when matched with an ultra-high surface capacity positive electrode of 30.06 mg cm-2(3.3 low N/P ratio).
The evolution of heat generation rate around lithium dendrites and its relationship with local dendrite growth were investigated based on a coupled electrochemical deposition-heat transfer model. Local rapid electrochemical deposition can easily lead to the accumulation of heat and the generation of temperature hot spots, which leads to the subsequent rapid growth of lithium dendrites, which in turn forms a more serious hot spot problem. The introduction of high thermal conductivity graphene layer on the surface of the diaphragm as an in-situ thermal diffusion medium can effectively eliminate the temperature hot spot, resolve the potential risk of rapid deterioration of dendrites, and obtain a more uniform lithium deposition morphology and stable and efficient electrochemical performance. This study provides a unique thermodynamic perspective for the in-depth understanding of the growth and evolution of lithium dendrites, and paves the way for the effective protection of lithium metal anodes and the practical application of lithium metal secondary batteries.
Related News






